Coronavirus: China looking at using stem cell therapy to treat severe cases
PUBLISHED MAR 5, 2020, 6:47 PM SGT
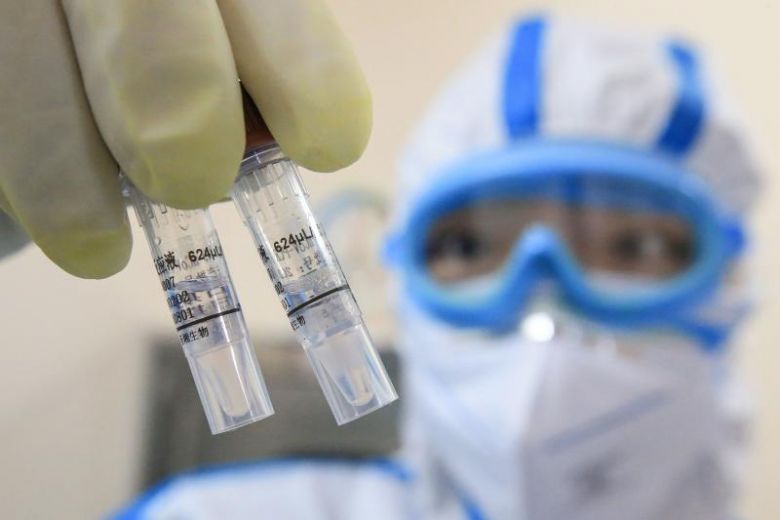
BEIJING (XINHUA) – Chinese researchers are studying the use of stem cell technology in the treatment of people critically ill with the coronavirus, the Science and Technology Daily reported. Four Covid-19 patients who received stem cell treatment while in a serious condition have been discharged from hospital after recovering, and the clinical trial of the therapy will be further expanded, Vice-Minister of Science and Technology Xu Nanping, was cited by the paper as saying.
Clinical Trial – Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With 2019 Novel Coronavirus
2019-nCoV infection has become an urgent public health event in China. As of 24:00 on January 26, 2020, there are 2744 confirmed cases and 461 severe cases in China, the number is still increasing. There is currently no vaccine and no specific antiviral treatment recommended for 2019-nCoV infection. About 20% of the patients were severe and some died of respiratory failure or multiple organ failure. Therefore, it is urgent to find a safe and effective therapeutic approach to pneumonia patients infected with 2019-nCoV.
The purpose of this study is to investigate safety and efficiency of MSCs in treating pneumonia patients infected with 2019-nCoV. This multi-center trial will recruit 20 patients. 10 patients received i.v. transfusion one round (3 times) of 3.0*10E7 cells of MSCs as the treated group, all of them received the conventional treatment. In addition, the equal 10 patients received conventional treatment were used as control. The clinical symptoms, pulmonary imaging, side effects, 28-days mortality, immunological characteristics (immune cells, inflammatory factors, etc.) will be evaluated during the 180 days follow up.
Japan responds: stem-cell therapy justified
Nature correspondence, published 30 APRIL 2019
Stemirac, stem cells from the patient’s bone marrow are cultured externally and then returned to the patient.
A double-blind study is therefore structurally impossible, and performing a sham operation on a control group would raise ethical issues.
In such cases, properly designed clinical studies can still test efficacy — as demonstrated for drugs approved by the US Food and Drug Administration as well as in Japan. Given the convincing response to Stemirac by the group of paralysed people under discussion, it could be unethical to withhold approval and deny treatment. The rationale for the safety, efficacy and quality of the product, and for the ethics of its approval, is given in the evaluation report by Japan’s Pharmaceuticals and Medical Devices Agency.
Full article here.
Mesenchymal stromal cell–based therapies for acute kidney injury: progress in the last decade
Fazekas B and Griffin MD. “Mesenchymal stromal cell-based therapies for acute kidney injury: Progress in the last decade”, Kidney International, In Press, 2020 (Review). 28 January 2020
In this review, we summarize the most significant new developments in the field of MSC-based therapies as they relate to AKI and reflect on the key gaps in knowledge and technology that remain to be addressed for the true clinical potential of MSC and, perhaps, other emerging cellular therapies to be realized.
Read the complete list of NEPHSTROM publications here: http://nephstrom.eu/nephstrom-publications/
Longeveron LLC Announces Completion of Enrollment in Phase 2b Clinical Trial of Longeveron Allogeneic Mesenchymal Stem Cells (LMSCs) in Aging Frailty
Longeveron LLC, a leading developer of adult stem cell technologies for aging-related and life-threatening conditions, announced today that it has completed enrollment in its Phase 2b Aging Frailty trial. This double-blinded, randomized, placebo-controlled study is designed to evaluate the safety and efficacy of LMSCs in patients with mild to moderate frailty.
This 150 subject multicenter study is sponsored in part by a Small Business Innovation Research (SBIR) Grant from the National Institute of Aging (NIA), as part of the Geroscience initiative focused on preventing and treating age-related conditions, functional decline, and disability.
Full article here.
